When choosing your form of plating, you need to know the differences in electroplating vs. electroless plating. Some projects will benefit more from electroless plating, also known as autocatalytic, than from electrolytic. Before you make a major decision on how your parts get finished, learn more about the coating options you have for a metal surface.
QUICK LINKS
What Is the Process of Nickel Plating?
What Is Electrolytic Plating?
Is Electroless Nickel Plating RoHS Compliant?
Advantages and Disadvantages to Electroless Plating
Advantages of Autocatalytic Plating
Disadvantages of Electroless Plating
What Are the Advantages and Disadvantages of Electroplating?
How to Determine the Right Plating for Your Project
Get Answers to Your Finishing Questions or a Quote From Us
What Is ENP Coating?
ENP coating is another name for electroless nickel plating, also known as an autocatalytic coating. Autocatalytic plating covers a base material in metal through a chemical reaction. A catalyst is something that starts a chain reaction, but with electroless plating, you don’t need to add anything to the solution to begin the process. It happens automatically, giving this finishing its name.
Typically, we use electroless plating for nickel finishing. Unlike electroplating, which requires a metallic base, we can plate any material with an electroless process. First, we carefully clean the part to remove any debris. Second, we dip the piece into a solution and add it to the mixture of anti-oxidation components.
What Is the Process of Nickel Plating?
During the process, the chemicals in the bath are unstable and deposit metal onto the base material. Some types of electroless plating require constant filtration of the solution to prevent debris from getting plated with the part. Nickel plating, however, does not need such strict filtering, nor does this process require electricity.
Several industries, such as the following, use electroless plating to attain durable, corrosive-resistant nickel coatings on their parts:
- Automotive Industry: Engine components that experience elevated temperatures and regular use require a sturdy exterior that part makers can get from electroless nickel plating.
- Manufacturing: Molds, machine parts, dies and more are commonly coated with nickel to resist rust and wear in any manufacturing setting.
- Aerospace Industry: Just like the automotive industry, the aerospace sector requires heat- and rust-resistant parts in the engine and throughout the aircraft. Nickel plating is one way to create the durability needed for these components.
- Petrochemical Industry: Petrochemical plants regularly need replacement valves, pipes, plugs and more. A long-lasting nickel coating withstands the heavy friction these parts may endure while protecting the components against corrosion.
Of the two types of plating, electroless is the most straightforward process. Because autocatalytic plating is not the best option for all projects, we also offer electrolytic plating.
What Is Electrolytic Plating?
Electroless plating does not require electricity, but electrolytic does. The electroplating process needs a conductive base, an electrical current and an electrolyte bath. We must thoroughly clean the base material before submerging it into the electrolyte solution. Along with the substrate, we plunge a bar of the metal used for plating. This metal, the anode, helps to replenish the metal ions in the bath as they deposit onto the base. Next, we connect both the base and plating metal to electricity. The positive wire connects to the anode while the negative wire attaches to the piece we plate.
As electricity passes through the metals and solution, it draws metal ions out of the solution and deposits them in a thin layer onto the base. The amount of voltage that passes through the solution depends significantly on the size of the part plated. Because the calculations for voltage are so exact and the time for electrodeposition is so fast, electroplating is not a task for amateurs.
Electroplating costs less than electroless plating does, and the price becomes a deciding factor for some. With electroplating, we can create thicker finishes than with electroless coating. How you may use electroplated materials, however, depends on what you require of the finished product:
- Matte Finish: For applications where the appearance is less important than the strength and wear resistance, choose the matte finish sulfamate nickel plating, which improves durability and strength.
- Bright Finish: If your project requires the aesthetic appeal of bright nickel, select sulfate nickel electroplating. Though it looks stunning with its gleaming finish, this coating does not provide the durability of sulfamate nickel. It also has a thinner depth compared to its matte finish alternative.
Is Electroless Nickel Plating RoHS Compliant?
The RoHS, Restrictions on Hazardous Substances, Directive placed tight restrictions on the amounts of dangerous materials that manufacturers could use:
- Lead: Lead, used in batteries and various electronics, cannot exceed 1,000 parts per million.
- Mercury: Mercury, found in lighting switches and automotive parts, must have levels that remain below 100 parts per million.
- Cadmium: Like mercury, cadmium needs to stay under 100 parts per million. Cadmium most often appears in batteries.
- Polybrominated Diphenyl Ether (PBDE): PBDE, an electronic flame retardant, must stay below 1,000 parts per million.
- Polybrominated Biphenyls (PBB): Like PBDE, PBB is a flame retardant for electronics that cannot exceed 1,000 parts per million.
- Hexavalent Chromium: Hexavalent chromium coats metals to prevent corrosion, but this substance cannot measure more than 1,000 parts per million.
At SPC, we use plating methods that comply with the most recent RoHS directive from 2013, which created the stiffest restrictions on chemical use. If you need RoHS compliant electroless nickel plating, we will fulfill your request.
Advantages and Disadvantages to Electroless Plating
Plating methods will have various advantages and disadvantages based on your project, what you need to plate and how you need the finished product to perform. Electroless plating may be a better option for specific projects, but its disadvantages may disqualify this finishing method from other jobs.
Advantages of Autocatalytic Plating
Electroless plating offers several benefits over electrolytic plating. Uniformity and the resources needed may make this a better choice for your project.
1. Uniformity
With electroless plating, the deposition may not be as thick as with electrolytic plating, but the coating deposits on the surface more evenly. Since the only factor in how thick the coating applies to the surface depends on the base’s contact with the solution, every surface has the same amount of metal coated to it. For an even finish, you cannot surpass the quality of electroless plating.
2. Durability
A layer of nickel plated onto an object with electroless plating provides superior strength for the item it covers. A 0.001-inch nickel coating lasted 1,000 hours in a salt spray chamber before forming red rust. This longevity is 10 times longer than chrome, which only withstands salt spray for 100 hours. To obtain similarly high corrosion resistance levels with nickel plating, choose an alloy with more phosphorous. The downside to increasing the corrosion resistance is a decrease in hardness. Balance these two qualities when selecting the amount of phosphorous in the nickel plating solution.
3. No Conductivity Required
Electroless plating does not require a conductive surface. Any properly cleaned surface can accept nickel plating through the autocatalytic process. By not requiring conductive materials, the range of options for electroless plating expands far beyond those for electroplating. The method of plating without electricity also removes the need for power, which can contribute to the complexity of the electroplating process.
Disadvantages of Electroless Plating
As many benefits as electroless plating has, this method will not suffice for all uses. The problems of this finishing technique may be minor inconveniences for you or issues that may require you to use electrolytic plating. Only your project parameters will help you determine the answer.
1. Limited Bath Life
During electrolytic processing, the metal in the solution constantly replenishes the metal ions in the liquid. Because electroless plating does not have a separate piece of metal in the solution, the bath will eventually run out of metal ions and need replenishment. Regularly monitoring the liquid and adding solution as required increases the cost and complexity of autocatalytic plating. If the lowest price is what you need, consider the advantages and disadvantages of electroplating.
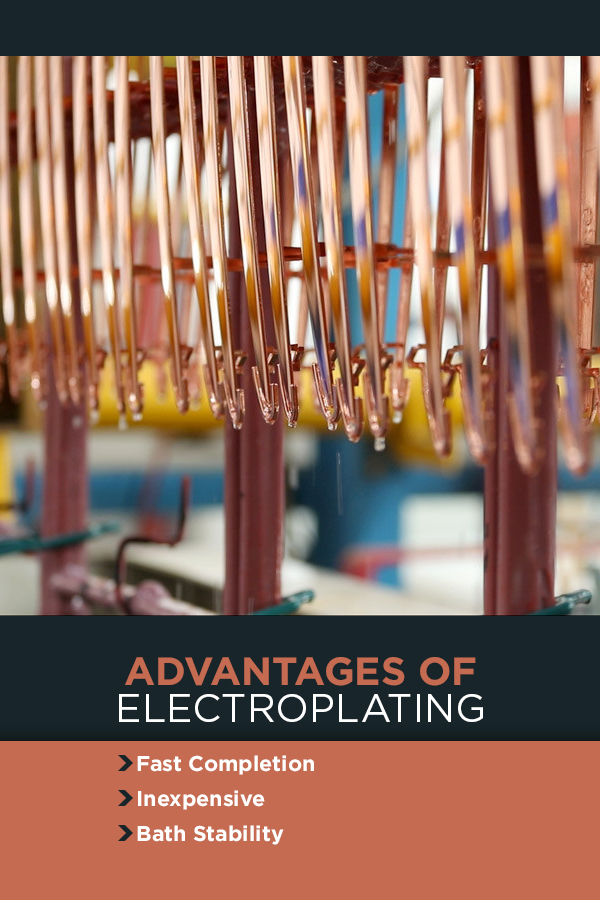
What Are the Advantages and Disadvantages of Electroplating?
Electrolytic plating works much better for specific applications. If time and money rank as your top concerns, you may find electroplating a better option. However, you will always need to balance the results with the cost.
Advantages of Electroplating
When time or your bottom line become deciding factors in selecting your project’s plating method, you may benefit from electroplating. This method gets results fast.
1. Fast Completion
The electroplating process happens in front of your eyes. Once you connect the power, the process begins. The exact speed depends on the metal concentration of the solution and the current strength. To make the electrodeposition process faster, increase the ion concentration or the power of the electrical current.
2. Inexpensive
Compared to electroless plating, electrolytic coating costs less. Though this process requires electricity, it does not need regular maintenance of the bath throughout the process. Additionally, some electroless plating solutions require heating, increasing the cost for utilities. The faster pace means plating more items at a time, reducing the cost of the plating facility’s time.
3. Bath Stability
Maintaining the electrolyte solution for electrolytic finishing requires less care than an electroless bath. The use of an anode in the bath replaces all metal ions lost from the plating process. Since the bath does not need constant care due to its chemical stability, an engineer does not need to use as much of his time for the process.
Disadvantages of Electroplating
While electroplating may sound great, it still has a few drawbacks. These depend significantly on the reasons you need an item plated. Not every downside to electrodeposition will apply to your project.
1. Limited Base Materials
Generally, electrolytic plating needs a conductive base. Some materials may accept graphite paint to allow electroplating, but the results will not be as tightly secured to the base material as electroless plating. If you need a metal coating on a ceramic or plastic base, electroless plating may be a better choice for you.
2. Coating Variations
Electroless plating creates an even coat perfectly every time. However, electrolytic plating can have areas of thicker deposits. Most notably, these denser areas occur on the corners of parts, where ions can collect and increase the amount of metal on the site.
3. Reduced Corrosion Resistance
Electroplated coatings cannot reach the hardness levels of electroless coatings. Compared to electroless, electrolytically plated nickel has less corrosion and abrasion resistance and lower surface hardness. The purity of the metal used in the process lies behind these attributes. In electroplating, 99-percent pure nickel coats the base. While this purity level gives adequate metallic coverage, it lacks the extra protection against wear found in nickel alloys with phosphorous.
How to Determine the Right Plating for Your Project
Selecting the best plating option for your project depends on multiple factors. You must consider your industry, required turnaround time and the substrate you will use to settle on the best plating method for you.
1. Industry
Multiple industries embrace both electroless and electrolytic plating. The best option for you depends on why you need a metal coating on an item.
- Durability: If you require extra strength, you will get better results from electroless nickel plating. Nickel-phosphorous alloys offer superior abrasion and corrosion resistance, especially when you choose a higher amount of phosphorous. For extra surface hardness, select lower phosphorous levels, which are inversely proportionate to hardness. Off-shore oil rigs exposed to constant sea salt spray is just one application that benefits from the high corrosion resistance of electroless nickel plating.
- Thickness: While we can control the depth of electroless coatings, electroplating offers a deeper metal finish on your project, though if you opt for electroless nickel plating with extremely low phosphorous levels, you can get a coating almost as thick as an electroplated surface.
- Cost: Does the cost matter exclusively? In many instances, electroplating costs less, but ask us for a free quote to get a proper estimate on the price. The price of electroless plating also varies based on the other metal used in the alloy.
- Appearance: Only with electroplating can you get a bright nickel coating on your parts. If you use the components in an area where appearance matters more than the coating’s wear resistance, choose bright nickel electroplating. Automobile bumpers and grilles are examples of when choosing to electroplate for presentation is the best option.
2. Turnaround
How quickly do you need the project completed? Electroplating requires less time, but you will sacrifice durability in exchange for that speed. If you don’t need the added wear resistance of electroless plating, choosing electrolytic finishing can get your project done faster.
3. Substrate
What lies beneath the surface of your metal finishing is as important for choosing a plating method as the metal used for coating. If you have a non-conductive base material, you may benefit more from electroless plating. If you must plate such products with electrolytic methods, the item will need a metal coating applied electrolessly first. This metal layer allows for the electrolytically applied coating to adhere to the surface. Without a conductive base, you will not be able to use electrolytic plating for your project.
What You Need to Know About Nickel Plating
Take a closer look at the various factors that can impact your decision-making process regarding electroless nickel and nickel electroplating:
- Electroless nickel plating vs. nickel plating: what’s the difference?
Get Answers to Your Finishing Questions or a Quote From Us
Electroless and electrolytic plating have advantages and disadvantages, and you must carefully weigh these against your project expectations to make the right decision. The parts you use will also help you choose the best plating method. Selecting the correct plating method will make the difference in the finished quality, time and cost. Don’t feel alone in your decision. At SPC, we have expertise in finishing to help you get the information you need to choose the method your project requires.
If you have questions about the various plating options, contact us at SPC. We also will give you a free quote for your project once you know which plating method will give your project the most benefits.