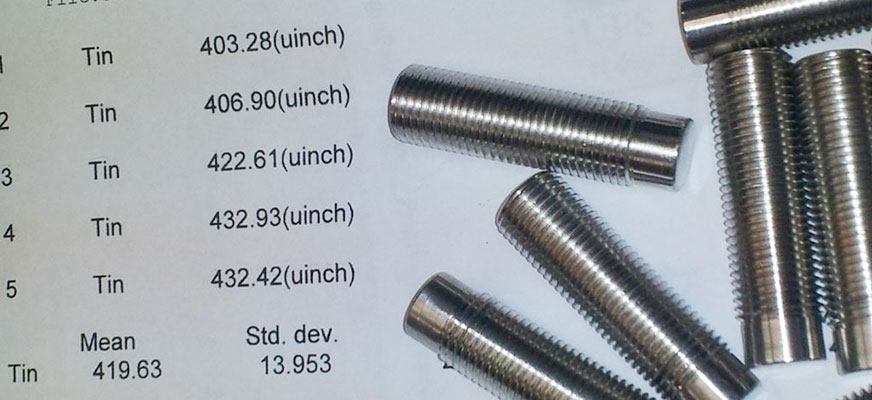
Tinning or otherwise regularly referred to as “Tin Plating“, is an ancient art form. This synthesis will cover contemporary and future uses of tin plating, strengths and weaknesses of the coating, best in practice tin processing and how to choose the right tin coating.
What Industries Make Use of the Electroplating of Tin?
The leading users of tin plating are divided between electronics, mechatronics, hardware, fasteners, food equipment and, most recently, solar. Here are a few examples from each industry: terminals and housings used in avionics, circuit breakers and interconnects used in construction of new homes and buildings, screws, nuts and bolts used on marine hardware in naval vessels, baking sheets and bowls used in food manufacturing and home cookware, and now solar panels.
Benefits of Tin Plating
Tin has many strengths making it a desirable choice due to its corrosion protection:
- Fretting, which means surface to surface corrosion.
- Environmental, which refers to sulfur bearing environments that cause tin to tarnish.
- Decent contact resistance and excellent solderability.
- Potential for mirrors like aesthetic.
- Flexibility to use matte tin plating to achieve a dull finish, or bright tin plating when a shinier finish is required
- No known toxicity to life on Earth.
- Tin plating is the lowest cost choice as compared to its rivals up the food chain such as silver plating and gold plating.
Drawbacks to the Electroplating of Tin
Tin’s primary weaknesses are its low temperature operating window of less than 450° F and inter metallic phenomena (i.e. tin whisker and dendritic crystal growths, or dendrites) of which the latter is understood while the anterior is not. Both cause severe damage to electronics by bridging and creating shorts in the electronics which they are assembled. There is no understood and agreed upon cause of the tin whisker type while dendrites migrate due to electromagnetic reactions of tin ions.
Reliable Procedures for the Electroplating of Tin
Following well known tin plating process procedures developed over the past several decades by numerous scientific institutions, for-profit chemical manufacturers and OEMs, below is a guide to minimizing and improving tin deposition:
- Pre-plating steps including under-plates.
- Under-plate metals such as copper and nickel are the most critical to achieve a great tin coating.
- Use “LESS” current density when processing through this pre-plate phase.
- Ensure that parts are as pristine as possible prior to the electroplating of tin. Having contaminates on the surface may cause poor deposit quality later on.
- Bake parts where necessary to remove excess hydrogen to promote optimal adhesion. When using nickel as an under-plate in particular, it is preferable that prior to plating you reduce the chances of intermetallic migration from occurring by baking the parts.
- During tin plating, use lower current densities, reduce or eliminate organics added to the bath (matte tin plating using a less luminous matte deposit is best) and deposit a minimum of 100µ inches (2.5µm).
- Reflow the tin coating using hot oils or heat lamps to imitate a hot dipped tinned part. This creates a longer shelf life to the coating to avoid the aforementioned weaknesses.
Tin Alloys
Tin has several alloys that are employed regularly in the tin electroplating process. These include cobalt, lead, nickel, silver (still in development) and zinc. The uses of nickel and cobalt have similar results when alloyed with tin. They increase hardness, reduce friction and can be used for a chromium alternative for both functional and decorative purposes. Lead helps to improve solderability. Zinc is alloyed specifically for increased corrosion protection and meant to be a cadmium replacement. By the addition of zinc up to thirty percent, you may also passivate the work with traditional chromates which add an added layer of atmospheric protection. Lastly, tin-silver is still being developed for multiple applications primarily for higher temperature solders as well as a hopeful alternative to pure silver plating for energy generation equipment.