
In the field of chemical sensor fabrication, material selection, precision and reliability are paramount. Using 3D printing technology with gold electroplating represents a transformative advancement. This integration combines the intricacy of components printed with 3D technology and the notable attributes of gold. Its rigor and versatility make this a valuable technique across various industries.
Find more statistics at Statista
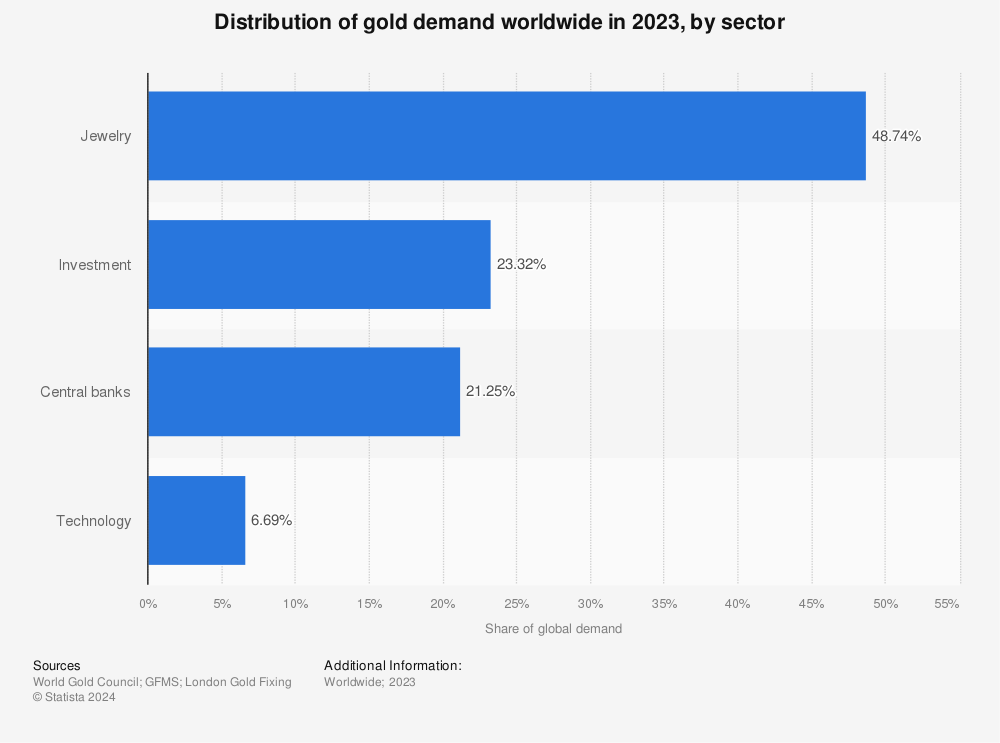
The technology sector is the fourth highest consumer of gold1, as this substrate aids component functionality. Due to gold’s high cost, the components themselves are not composed of it but rather gold plating. The thin layer of gold plating protects components from heat, corrosion and wear while facilitating a stable electrical connection.
The synergy between 3D printing and gold electroplating allows researchers and engineers to fabricate custom-made chemical sensors2. Whether it is for the application of monitoring biochemical reactions, ensuring food safety or detecting environmental pollutants, these sensors offer an efficient and versatile solution.

An Overview of Gold Electroplating
The electrodeposition or electroplating process3 deposits a metallic layer onto a substrate’s surface using a direct current (DC). In all electroplating processes, an electrical current passes through an electrolyte solution to facilitate the transfer of metal ions from an anode to the substrate, resulting in a bonded metal layer. Electroplating enhances the durability of objects to which it adheres. The electroplated component also shows resistance to environmental actors.
As “Au” is the symbol for gold on the periodic table of elements, the gold electroplating process is also referred to as Au plating or Au electroplating. In preparation, the gold part is polished to smooth out rough edges and even out the surface. Next, a metal salt is created by combining an acid and base to get a neutralization reaction. This salt is added to water to make up a bath for the electroplating component.
The Process of Gold Electroplating
Gold electroplating starts with chemical preparation by way of a gold plating bath. The process follows the dissolving of metal salts into a solvent4 to facilitate plating, which starts at the cathode as hydrogen ions reduce to hydrogen gas. The surface and preparation of the electroplating solution are critical success factors.
Several plating techniques for different applications create the bond between gold electroplating and various components:
- Reel-to-reel gold electroplating: This continuous electroplating method uses reels of raw material5 before it is molded into components. It can cover the entire substrate or just specific parts.
- Gold rack electroplating: This is an adept solution for fragile components and complex projects6 where the substrate hangs on a rack, which is lowered into the electrolytic bath.
- Gold barrel electroplating: This quick and economical plating solution7 applies the coating to the substrate in a uniform fashion. It only works in applications where the entire surface needs plating.
- Gold immersion plating: This method does not involve an electrical current. The substrate is dipped into an electrolyte solution8 containing gold ions, which produces a thin coating similar to the properties of electroplated gold.
Gold plating 3D prints is a highly controlled and reliable process where a thin layer, often about 0.0002 inches of gold9, is applied to a substrate’s surface to utilize the metal’s exceptional corrosion resistance and electrical conductivity. The chemical bond that forms is permanent, ensuring the gold plating cannot detach from the surface of the base metal, which is often brass, nickel, silver or copper.

Benefits of Gold Electroplating
Industries that make use of gold electroplating 3D prints include automotive, electronics, medical, aerospace, and oil and gas. Gold electroplating offers many benefits, such as:
- Corrosion resistance: This metal does not react with oxygen, and this corrosion resistance facilitates a strong connection between electrical components over a longer period or time.
- Heat protection: Gold’s melting point is 1947.52 degrees Fahrenheit10. It conducts heat well, which is particularly helpful in technology.
- Reflectivity: Gold can reflect high amounts of ultraviolet radiation, keeping electronics at cool temperatures. These reflective properties are specifically essential in applications like satellite designs and spacecraft.
- Expanded connection area: As gold is ductile and malleable, it offers an opportunity to create an expanded connection area.
- Longevity: The combination of these properties gives gold greater wear resistance, offering longer protection to the layers beneath it.
Types of Gold Plating Baths
Gold plating baths are integral components of the gold electroplating process. The three primary types of baths are alkaline, acid and neutral, defined by their unique chemical compositions and pH levels, which determine the properties and characteristics of the deposited gold layer.
The conductivity measurement uses Faraday’s law of electrolysis11, which describes the quantitative relationship between electric currents passing through the electrolyte and substances produced during the electrolysis process. Understanding these processes and their advantages is paramount to achieving the desired outcomes in functional applications.

Alkaline Gold Plating Baths
Gold plating via an alkaline bath offers lower internal stress to the substrate. The ingredients for this bath are gold cyanide, usually potassium gold cyanide and alkaline buffer to maintain pH, and alkaline metal cyanide, such as sodium cyanide. Using potassium gold cyanide increases the level of purity in the gold layers. This bath’s pH ranges between 9 and 1312 and generally produces fine-grained, highly uniform gold deposits.
- Surface preparation: The substrate undergoes meticulous surface preparation and cleaning to ensure proper adhesion.
- Bath preparation: The bath is prepared with specific concentrations of gold and cyanide compounds and often contains additives that control the plating properties.
- Electrolysis: The substrate connects to the cathode and the negatively charged electrode and is immersed in the bath with a pure gold anode. A DC current is applied to encourage gold ions from the bath to migrate to the cathode’s surface to form a layer of pure gold.
- Parameter control: The outcome depends on parameters like current density, temperature and plating duration. Fine-turning these parameters helps ensure the gold layer’s desired quality and thickness.
Acid Gold Plating Baths
Acid baths produce bright, highly adhesive gold deposits that align well with many applications. This bath type uses gold chloride or sulfate as the source of gold ions. It is typically acid, using sulfuric acid to maintain the acidity, with a pH level between 3 and 612. Depending on the bath composition, it offers a range of gold finishes, including matte and high-gloss.
- Substrate preparation: Similar to alkaline gold plating baths, the substrate undergoes a thorough cleaning and surface activation.
- Bath preparation: Using concentrations with adequate combinations of additive and gold compounds helps achieve the desired finish. The right concentrations ensure no burning, which happens when an electroplating solution is prepared with the wrong quantities of substrates or temperatures.
- Electrolysis: In this case, the substrate functions as the cathode, using an inert anode made from platinum or titanium. The DC current oxidizes metal atoms in the gold, dissolving them into a solution. This process encourages gold ions to move from the bath to the cathode, where they reduce to form the gold layer.
- Parameter control: The gold deposit’s thickness and quality result from temperature, current density and plating time. It is worth noting that this gold plating bath yields the purest commercial gold deposit.

Neutral Gold Plating Baths
Neutral baths are versatile enough to offer a variety of gold deposit properties and are used for plastic or ceramic substrates. They contain a mixture of gold sulfate and gold chloride with additional compounds to keep a neutral pH level between 6 and 812. Complexing agents like ethylenediamine and thiourea are added to the bath to stabilize the gold ions, which facilitates precise control over gold deposition.
- Surface preparation: A clean surface is essential for successful plating and adhesion, so thorough substrate preparation is critical. Keeping plating tanks free of contaminants is also vital to ensure high-quality application.
- Bath preparation: The bath uses no free cyanide as the compound is unstable in a neutral pH range.
- Electrolysis: The substrate serves as the cathode, with a platinum or titanium anode also used in acid gold plating baths. A DC current facilitates dissolving gold ions, which reduces at the cathode before they are deposited onto the substrate.
- Parameter control: Similar to other plating methods, controlling parameters is vital to achieving the correct layer quality and density.
Benefits of Gold Plating for Chemical Sensors
Gold electroplating provides multiple advantages for chemical sensors and other electronic devices. Gold is a highly durable yet malleable material and can be easily shaped into thin wires and sheets for electronics, like chemical sensors. It is also an excellent conductor of heat, electricity and light.
Electronics manufacturers often use gold plating to make circuit boards more conductive and corrosion-resistant. A strong circuit board connection is critical for any electronic device. Since gold is nonmagnetic, it will not disrupt electrical signals. These characteristics make gold plating a prime solution for chemical sensors.
Using gold plating in conjunction with plastic also offers many benefits. Plastic offers a balance of properties like chemical resistance, electrical insulation and mechanical strength to complement the manufacturing of chemical sensors.
In electronics, gold’s appeal lies in its functionality and how it works to ensure the longevity of electrical components. Gold-plated components allow chemical sensors to deliver superior performance across various applications, including health care diagnostics, environmental monitoring and scientific discovery.

Using gold-plated components for chemical sensors yields the following benefits:
- Electrical conductivity: Gold has a low electrical resistance, making this metal ideal for ensuring efficient signal transmission in sensors. Gold-plated components gain this conductivity, adding to chemical sensors’ performance. The low resistivity facilitates quick and accurate electrical signal measurement from chemical reactions.
- Biocompatibility: Chemical sensors in medical or biological applications require biocompatibility. Gold is nontoxic and biologically inert, which ensures safety in sensor contact with biological samples. These components also pose no risk of adverse reactions or cross-contamination when integrating diagnostic tools or medical devices.
- Chemical inertness: In chemical sensor applications, interference from sensor materials can lead to inaccurate readings. Gold’s chemical inertness ensures it does not react to substances, which preserves the integrity of measurements. This quality transfers to components coated with gold plating, making this advantageous in analytical chemistry.
- Signal-to-noise ratio (SNR): SNR is critical for accurate detection and measurement in chemical sensing applications. Gold plating reduces electrical noise and signal interference, allowing sensors to discern minute changes more precisely.
- Increased sensitivity: The synergy in gold’s conductivity and corrosion resistance creates a heightened sensitivity in chemical sensors. This sensitivity allows sensors to detect subtle variations in chemical parameters, an invaluable attribute to environmental monitoring and industrial quality control applications.
- Microfabrication compatibility: Gold-plated plastics can be integrated into microelectromechanical systems and microfabrication processes where miniaturized chemical sensors function.
An Overview of 3D Printing
The field of analytical chemistry relies on chemical sensors’ efficiency, simple integration and cost-effective benefits in various sectors. 3D printing brings the flexibility and innovative design solutions needed to this manufacturing process. There are several types of 3D printing that may be utilized in this process:
- Fused deposition modeling: Thermoplastic filaments are used to form a three-dimensional object in a layer-by-layer process. The versatility afforded by this method simplifies assembly by allowing sensor components, like temperature sensors, strain gauges and piezoelectric sensors, to be added to the printed structure.
- Selective laser sintering: A high-power laser fuses powdered materials like polymers or metals. This method can create a more durable structure with complex geometries that are well-suited for aerospace applications.
- Stereolithography: Well known for precision and smooth surface finishing, this resin-based 3D printing method uses an ultraviolet laser to cure liquid resin into solid components.
- Direct ink writing: This additive manufacturing technique uses soft, flexible materials like conductive inks and elastomers. It is often employed in creating stretchable sensors for wearable technology and soft robotics.
- Multi-material 3D printing: This technique combines different materials into a single print for multi-functional sensors. It can include rigid and flexible elements along with conductive and nonconductive materials to reduce manufacturing steps.
3D Printed Chemical Sensors
These electrochemical sensors are designed to detect and quantify specific chemical compounds or ions13. Through a series of electrochemical reactions, they convert chemical information into electrical signals. What sets them apart is that they are made in a single step using 3D printing technology, which creates complex, tailored structures with microscale precision. Sensing-based devices made with the help of 3D printing use a combination of conductive and nonconductive thermoplastics.
One advantage of 3D printed chemical sensors is their customizable nature, which allows additional features like microfluidic channels or multi-sensor arrays to be added to the component. These devices offer a new dimension of adaptability, integrations and precision to technological uses in various fields, like science, manufacturing and electronics.

3D Printed Sensor Applications
3D printing simplifies a historically complex manufacturing process in sensor fabrication. This technology produces components faster at a lower cost and more accurately than traditional methods. The additive nature of this printing technology creates the opportunity for intricate sensor designs with rapid production of prototypes, which enables the development of tailored sensing solutions for various applications:
- Medical diagnostics: Chemical sensors are incorporated into diagnostic devices, detecting pathogens, biomarkers and other specific molecules in DNA. These sensors’ accuracy and rapid response enable early detection of anomalies alongside personalized treatment plans.
- Environmental monitoring: Precise and timely data collection enables engineers to assess air, soil and water quality. 3D printed sensors with gold electroplating can integrate into remote locations to detect heavy metals, pollutants and other chemicals to provide valuable data for environmental conservation efforts.
- Industrial process control: To adhere to industrial applications’ safety standards and quality control, 3D printed sensors aid in real-time process monitoring. These devices pick up variations in chemical concentrations, leading to automated adjustments that ensure consistent product quality.
- Aerospace and defense: These sensors are compact, lightweight and rugged enough to monitor critical parameters like pressure, temperature and chemical composition. 3D print electroplating features in these sensors amplify the sensor’s reliability in challenging environments.
- Wearables and Internet of Things (IoT): In the era of wearable technology and IoT, these low-power sensors can integrate into everyday life. Wearable devices can monitor environmental conditions, detect allergens and analyze sweat for health insights.

The Future of 3D Printed Sensors
Data collection, analysis and interpretation are increasingly important, securing the future of chemical sensing in connection with 3D printed gold electroplating, promising heightened performance and accuracy. Sensor design and application are morphing into their own faculty as smart sensor development and intelligent domotics concepts start to flourish. One study suggests that in the future, 3D printed plasma sensors or retarding potential analyzers can drive improvement regarding weather research14.
Students at the Massachusetts Institute of Technology have begun developing a system that incorporates sensors into the 3D printed design15. By integrating sensors with a rotational mechanism, this prototype aims to eliminate some manual processes in manufacturing through automation. The road ahead is paved with innovation, holding much promise for more versatility, precision and efficiency in chemical sensor manufacturing.
Request a Quote From SPC Today
Gold is known for its exceptional durability, conductivity and corrosion resistance. 3D printed gold electroplating for chemical sensors offers an unparalleled opportunity for engineers to enhance sensing capabilities. The method harnesses the precision, speed and unwavering quality of 3D printing technology to manufacture an intricate sensor structure, while gold electroplated plastic components add a highly conductive, durable surface.
The endeavor of 3D printing, fused with gold electroplating for chemical sensors, is driven by a pursuit of customization and precision. At SPC, we have perfected our gold plating techniques over the span of more than 80 years. While 99% of our gold plating work16 serves the electronics industry, we offer industrial plating and metal finishing services to a variety of industries.
Partner with SPC for a cost-effective solution to plating semiconductors. Get started now by requesting a free quote17 or contacting us for more information today.
Sources:
- https://www.statista.com/statistics/299609/gold-demand-by-industry-sector-share/
- https://www.sciencedirect.com/science/article/pii/S1388248120301788
- https://www.sciencedirect.com/science/article/abs/pii/S0272884219320127
- https://www.sciencedirect.com/science/article/abs/pii/S0257897222010702
- https://nmfrc.org/pdf/sf2002/sf02l01.pdf
- https://iopscience.iop.org/article/10.1088/1742-6596/1378/4/042084/pdf
- https://www.sharrettsplating.com/plating-methods/barrel-plating
- https://www.ipc.org/TOC/IPC-4556.pdf
- https://www.sharrettsplating.com/blog/how-gold-electroplating-is-done/
- https://www.rsc.org/periodic-table/element/79/gold
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Faraday’s_Law
- https://www.sharrettsplating.com/blog/choosing-the-right-gold-plating-bath/
- https://www.mdpi.com/2227-9040/7/3/39
- https://www.techbriefs.com/component/content/article/tb/pub/briefs/manufacturing-prototyping/47536
- https://www.allaboutcircuits.com/news/mit-creates-new-way-to-incorporate-sensors-into-3d-printed-designs/
- https://www.sharrettsplating.com/coatings/gold
- https://www.sharrettsplating.com/quotes/




