UPDATE: (8/24/2021) SPC no longer offers brush plating services. This blog is for educational purposes only.
Please refer to our coatings, base materials, or plating methods pages to learn more about our services.
Metal plating has been used for hundreds of years to provide objects and mechanical components with additional and desired properties of a specific metal. Depending on the preferred outcome, different types of metal or alloys including copper, aluminum, tin, gold, cadmium, rhodium, zinc, silver, nickel or chromium may be used in the plating process.
The reasons for plating a component or object in metal can range from just adding a nice, metallic aesthetic to a decorative object or a need for the essential physical, mechanical and chemical properties of the specific metal or alloy for performance purposes. Improved corrosion resistance, strength, durability, reduced friction, increased solderability and even changes to conductivity might all be factors in selecting a component for the plating process.
Whatever your reasons for selecting a component for metal plating, the process can usually be separated into two different types of processes: electroplating or electroless. Electroplating demands the use of an electric current, while electroless methods are the result of an auto-catalytic chemical reaction. Each method offers their own unique advantages and disadvantages, but either method allows for a coating of metal that will provide the selected component with important aesthetic and new physical properties.
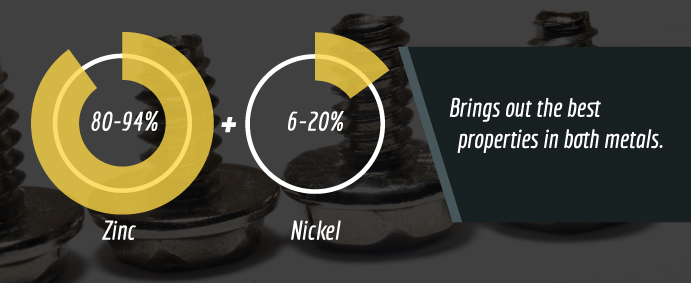
In some cases, combining two or metals to create an alloy can offer advantages as well maximize the desired benefits of each metal selected. This can also help reduce costs without sacrificing quality. For example, when trying to improve corrosion using a zinc-nickel alloy with approximately 80-94 percent zinc and 6 to 20 percent nickel brings out the best properties in both metals. The addition of nickel slows the corrosion process. Zinc-nickel coated parts can help prevent the formation of white rust for up to 500 hours, and up to 1,000 hours for red rust during salt-spraying testing.
Electroplating and Electroless
Before we take a look at more specialized methods of metal plating, understanding the general process of both electroplating and the auto-catalytic chemical process of electroless methods will help better differentiate between the two.
Electroplating requires the use of an electric current, which is used to reduce dissolved metal cations, which are ions with a positive charge. The electric current allows the cations to form a thin metal coating onto the object in a process called electrodeposition. Electroplating allows for the change of surface properties of the selected object, making it suitable for increased corrosion resistance, reduced friction and other needed qualities which will help improve the durability and performance of the part.
Just like a circuit, the electroplating process relies on different components, or electrodes, to achieve the thin metal coating. The item selected for plating is the cathode — the negatively charged electrode, while the material or metal used makes up the anode — the positively charged electrode. Both components are immersed in an electrolyte bath containing metal salts and other ions to allow for the proper flow of electricity.
This is not the same technique used in electroless immersion as the external power supply must create the direct current to the anode to oxidize the metal atoms and dissolve them into the electrolyte solution. These dissolved metal ions in the solution are then reduced and begin to plate the object. The metal used for the anode continuously replenishes the ions in the electrolyte.
After the process is completed, the chemical, physical and mechanical properties of the object you selected for plating will be altered. For example, tensile strength and surface hardness might add the strength needed for a tool or part that could not perform under pressure prior to plating. By adding metal plating, you can also prevent dulling of the tool and reduce corrosion, which provides longevity.
Electroless methods do not require the use of external electricity, but instead depend on an auto-catalytic chemical reaction. This process usually involves creating several reactions at once within a liquid solution where the component is immersed. These chemical methods force the desired metal to plate the object. Unlike electroplating, which requires two electrodes, electroless only uses one and does not rely on any external power source.
Instead, a reducing agent is used. One of the most common metals used in this type of plating is nickel, but other metals like gold, silver and copper can also be applied to components through electroless methods. One of the major benefits is that it is usually less costly because electrolyte baths and external power sources are not needed, which can help lower expenditures. In addition, electroless methods allow for flexibility in the size and shapes of objects that can be plated, but unfortunately, they are much slower and cannot achieve the thickness electroplating can.
In addition to lower utility bills, electroless makes it easier to achieve an even coating on parts and offers more flexibility in terms of volume and varying levels of finish brightness. As with electroplating, the chemical, physical and mechanical properties of the completed work piece will be altered, adding enhanced durability to the component.
What Is Selective Plating and Brush Plating?
When metal plating required in more localized areas. This process, which is related to electroplating is often referred to as selective plating, or brush plating.
The brush, which is usually a stainless steel body wrapped in cloth, holds a plating solution and inhibits the item from making direct contact. Through the use of low voltage, the brush dipped in plating solution allows for localized plating by an operator. Skilled operators can use this selective electroplating service to apply an even distribution of plating material across the items localized area. Brush plating allows for spot-plating techniques, making it useful in both the repair and refurbishment of parts and components. Unlike full electroplating techniques which require an immersion in an electrolyte bath, selective plating allows the operator to target a specific area using a plating solution of electrolyte and anode connected to a wire.
In many ways, brush plating may appear similar to welding when compared to other forms of electroplating because it uses a flexible and maneuverable brush attached to a power supply. The cathode is still the component you have selected for plating, but the anode is now connected to a handle and wrapped in an absorbing material, usually cloth. The cloth absorbs the electrolyte during the brush plating process.
As the operator moves the anode over the cathode, it completes the circuit and supplies the electrolyte continuously. The electrolyte can be supplied via a pump or by dipping.
The Advantages of Brush Plating vs. Immersion Washing
One of the greatest advantages that brush plating services, or selective electroplating services, offer over a traditional immersion bath — or tank plating methods, is flexibility. The equipment for brush plating is mobile and can be done anywhere — from the workshop to onsite at a customer’s location without requiring the transportation or shipment of heavy and delicate components.
This is ideal and much faster than traditional electroplating techniques. It allows for the service, repair and refurbishment of parts quickly. In addition, it may also reduce the need for machining since metal can be deposited onto a component in thin layers. While there are no limitations such as the level of thickness that can be achieved, brush plating is not as economical as traditional electroplating and electroless methods in high volume.
Selective plating is ideal for small low plating services with low volume needs. In addition, while immersion methods are usually more hands off once the process begins, there is a need for more involvement from trained operators for brush plating.
Quick Guide to Brush Plating Advantages vs. Immersion
Here’s a quick look at the advantages of brush plating when compared to immersion:
- Mobile services that can be performed anywhere
- Faster method for small localized areas
- Reduces the need for masking
- Ideal for large parts not suited to immersion baths
- Reduces high chemical volumes needed for immersion plating
- Reduces overall utility expenses and requires less electrical power
While brush plating offers many advantages, electroplating via immersion bath is also more suitable to large-volume projects and offers thickness not achievable through selective plating methods. Electroplating offers improved surface uniformity, protection from surface abrasion and the ability to build the thickness of the metal substrate. This is ideal for creating adhesion for components that need to be painted as surfaces can be enhanced through electroplating.
Depending on the scale or demands of your project, electroplating may be a more economical alternative to brush plating.
What Is Selective Plating Used For?
Selective plating is a great service that offers flexibility in a variety of industries for varying types of components. Whether you work in the energy industry, aerospace or manufacturing, selective, brush plating methods can be used to eliminate down time and keep your tools and machinery operational.
One of the best uses for brush plating is to eliminate build up on surfaces, which includes essential bearings and bushings. For areas with close tolerances and wall thicknesses, selective plating methods can be used to plate a localized area. In addition, certain types of metal offer unique advantages when it comes to adjoining parts in soldering and welding applications.
Selective plating of a metal that offers better physical and mechanical properties at joints can be applied for easier solderability or welding as well as for increased strength at component joints. In some cases, enhanced conductivity might be desirable, but not economical across the entire surface area. Brush plating is often used to add these desired properties for electrical components that rely on precious metals. This can help reduce expenses from more traditional methods and the amount of material used.
Some components may also be too large or be oddly shaped, making them unsuitable for an immersion bath. By using brush playing, complex components can be plated quickly and efficiently. Selective plating also offers increased flexibility for the plating of components that are required for critical applications.
Common Types of Metal and Alloys Used in Plating
Several different types of metals and complex alloys can be used with electroplating, electroless and selective plating services. Each metal and alloy offers their own chemical, mechanical and physical properties. In some cases, a nice finish brightness and clean aesthetic may be wanted, while other times components will need to be plated to withstand stresses that come from wear and tear.
Here is a look at a few of the common metals used in the plating process:
- Copper. Copper, and its alloys, are some of the most common types of metals used in plating because of their affordability and high electrical conductivity. Copper is often used for the manufacturing of electronic components and circuit boards. Because of its high plating efficiency, and its low cost, copper is one of the least expensive metals used today. In addition, copper’s corrosion resistance is essential to heat transfer applications, and while stainless steel is the most affordable alternative, it only offer 1/30 the thermal conductivity of copper.
- Zinc. The U.S. pennies you carry around in your pocket are a direct result of zinc plating as zinc is used to coat the thin copper in every single penny. Zinc resists oxidation and corrosion, making it one of the most commonly used metals in automotive components as well as fasteners and nails. It is often used in the galvanizing process through electroplating.
- Nickel. Electroless nickel plating is very common. The metal is used in a variety of objects we utilize everyday including kitchenware, bathroom and plumbing fixtures, decorations and doorknobs. Nickel and its many alloys are resistant to wear and make it one of the most widely used metals in the plating service industry.
- Gold. No other metal is quite like gold, which is sought after for its high resistance to oxidation and high electrical conductivity. Unlike copper, though, gold is much rarer and more expensive. Because of its higher material cost, gold plating is often reserved for only small components when it is needed. However, gold plating is often used for the creation of jewelry and for electronic components such as connectors because of its unique mechanical and physical properties.
- Chromium. Because of its surface finish, chromium is often used for decorative purposes, but it also is advantageous for reduced friction and resistance to corrosion. For decorative pieces in automotive components, chromium is often used for plating, which is done through electroplating in an electrolyte solution.
- Silver. Similar to chromium, silver is often used for decorative purposes, but like other metals, it offers enhanced electrical conductivity. Silver is less costly than gold, but it still retains a higher material cost than other metals. Silver also does not perform well in certain applications and has mechanical and physical properties that may not hold up in the long term compared to other less expensive metals and alloys.
- Cadmium. One of the best metals used for plating components that need enhanced paint adhesion is cadmium. Cadmium also offers increased corrosion resistance and provides benefits for long-term wear protection with less material usage. In addition, cadmium can be plated onto almost all conductive metals, making it ideal for numerous industries.